About the study
Participant information statement
HREC Project Number: Mercy Health 2021-007, Curtin University 2021-0315
Project Title: Therapeutic ultrasound treatment for women with inflammatory conditions of the lactating breast
Chief Investigator: Dr Leanda McKenna, Curtin University
Student researcher: Emma Heron (PhD candidate)
Version Number: 6
Version Date: 05/08/2022
What is the project about?
Inflammatory Conditions of the Lactating Breast (ICLB), such as engorgement, blocked duct, and mastitis, commonly occur in the early weeks and months after birth. Therapeutic ultrasound is the most common treatment used by Australian physiotherapists for ICLB, however no large clinical trial has looked at the effectiveness of ultrasound treatment for ICLB. Currently, physiotherapists do not know which ultrasound settings work best, so this study aims to compare different ultrasound settings for ICLB. This is important in helping to improve care for women with ICLB. We will be recruiting 160 women to take part in this trial.
Who is doing the research?
The project is being conducted by Emma Heron (Curtin University), as part of her Doctor of Philosophy, and supervised by Dr Leanda McKenna (Curtin University), Dr Adelle McArdle (Monash University) and Professor Donna Geddes (University of Western Australia), with clinical collaborator, Melinda Cooper. There will be no additional costs to you, and you will not be paid for participating in this project. If you are attending through your agreed private physiotherapy provider for treatment, there is a $20 rebatable private health insurance cost.
Why am I being asked to take part and what will I have to do?
You have been asked to take part in the project because you have the condition we are researching; an ICLB.
You will receive:
· Up to 3 consecutive days of ultrasound treatment to your affected breast, which could either be high intensity ultrasound, low intensity ultrasound or no intensity ultrasound. This will take place at your agreed private physiotherapy provider, Mercy Hospital for Women, or at your home.
· Standardised education and advice consisting of the common comfort measures outlined in the literature that are helpful to soothe inflammatory breast symptoms. This will be delivered via:
1. A video during your first ultrasound treatment
2. An infographic handout for you to take home
3. Copies of the video and infographic on the trial’s website.
We ask that you adhere to these comfort measures and not try different treatments or change what you are doing during the trial (for example, stopping or starting the use of heat, ice, anti-inflammatories).
Your first day of treatment will be prioritised for the same day as recruitment, or as soon as appropriate. Before the ultrasound treatment you will be asked to make the breast as soft as possible, either by breastfeeding or expressing. We will then:
· Measure the size of any breast hardness area using cling wrap
· Collect a breast milk sample of up to 5 ml from your affected breast to measure indicators of inflammation (via hand expression or use of an electric pump, with strict hygiene and sterilisation protocols in place to minimise risk of infection)
· Ask you 8 questions relating to the severity of your symptoms using an ICLB specific validated questionnaire.
· Ask you 1 question about whether your condition has improved or deteriorated over the course of the trial.
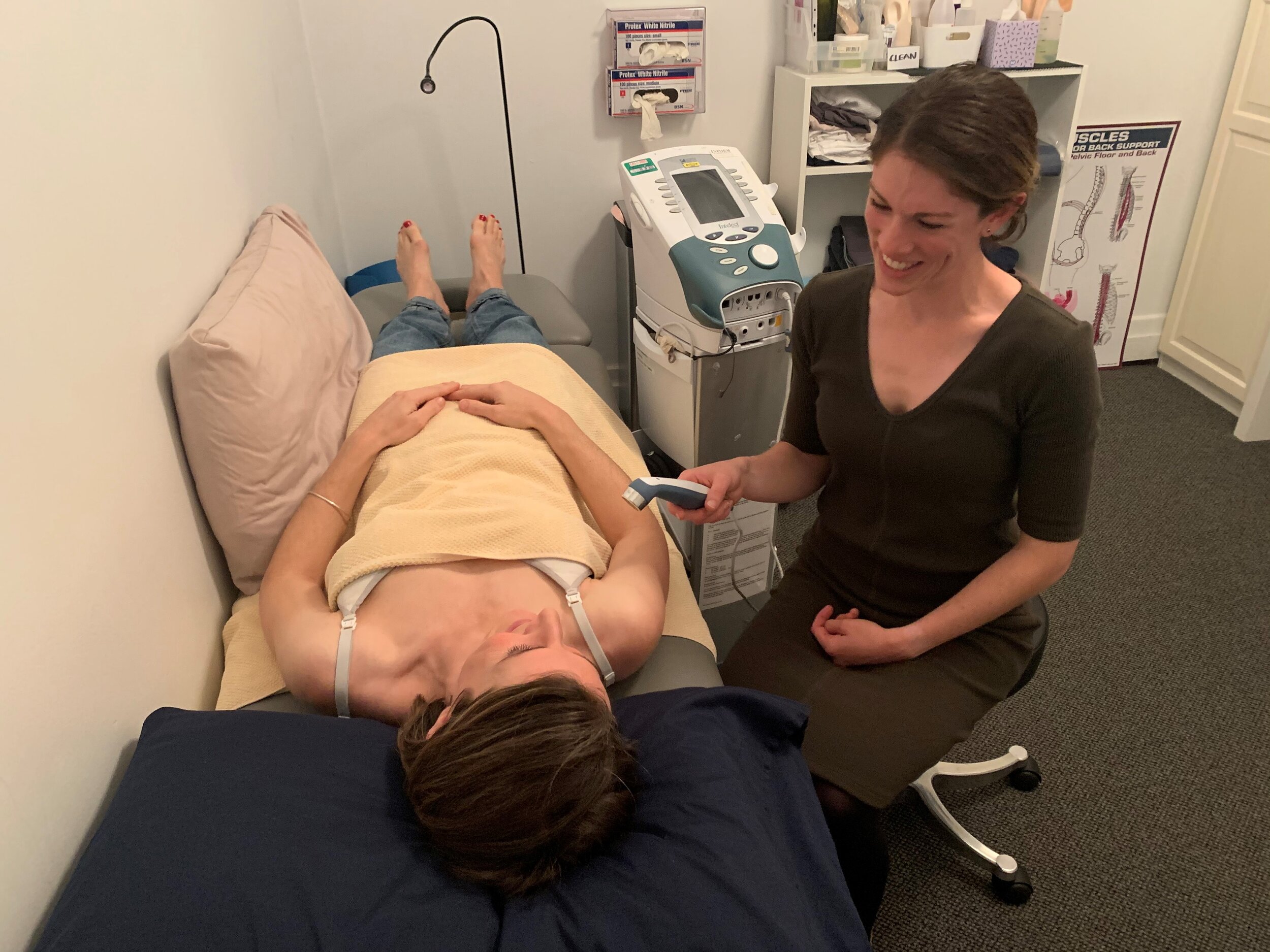
For the ultrasound treatment, you will be asked to remove all clothing covering the breast, and will be positioned lying down, appropriately covered with towels. You may receive 10 to 20 minutes of ultrasound treatment to your affected breast.
You will be asked not to inform the researcher if you feel heat from the ultrasound head unless it becomes more than a comfortable warmth.
You will be encouraged to breastfeed as soon as possible following the ultrasound treatment. We will require approximately 1 hour of your time for the first treatment session, and approximately 45 minutes of your time for each subsequent treatment session. Your baby can attend these sessions.
After each ultrasound treatment, we will ask you questions about the standardised education and advice, such as which comfort measures you have been using and any additional treatments or things you have used or trialled. This will be done via an online survey, either using your smart device or the researcher’s tablet. You will be given a QR code or email link to access your questionnaire. One week after the 3 days of ultrasound, we will:
· Re-measure the size of any breast hardness area using cling wrap
· Collect another breast milk sample of up to 5 ml to measure indicators of inflammation
· Ask you 8 questions relating to the severity of your symptoms using the ICLB specific validated questionnaire
· Ask you 1 question about whether your condition has improved or deteriorated over the course of the trial
· Ask you 4 extra questions about your satisfaction and experience with the ultrasound treatment.
This final session will all take place at your home or a mutually convenient location and will require approximately 20 minutes of your time.
We may contact you in 3 and 6 months, via email or phone, to ask you a few questions about the longer-term success of the treatment.
Are there any benefits’ to being in the research project?
You will receive one of the 3 ultrasound treatments that physiotherapists currently offer when treating ICLB. This will be provided by an experienced women’s health physiotherapist who works at both Inform Physiotherapy and Mercy Hospital for Women. This research also gives you the opportunity to discuss your opinions on your satisfaction and experience with the ultrasound treatment; sometimes, people appreciate this opportunity.
We expect the results of this research will improve care for women with ICLB.
Are there any risks, side-effects, discomforts or inconveniences from being in the research project?
Risks or side-effects of ultrasound are minimal but include possible risk of overheating, as with usual ultrasound treatment. To minimise overheating:
· The ultrasound will be applied by the physiotherapist with training and expertise in the use of ultrasound
· They will test your sensation, or ability to tell the difference between hot and cold on your breast, before applying the ultrasound
· You will be given an ultrasound safety summary sheet and asked about comfort throughout the treatment.
· The physiotherapist will be able to decrease the intensity on the machine if you report more than a comfortable warmth.
Given ICLB can rapidly progress in severity to a serious illness, you will be given the standardised education and advice, and asked to perform these comfort measures. You will also be encouraged to seek medical care at any time throughout the trial if your symptoms are worsening or not improving.
We have been careful to make sure that the questions in the survey do not cause you any distress. But if you feel anxious about any of the questions you do not need to answer them. If the questions cause any concerns or upset you, please see your GP or access Perinatal Anxiety & Depression Australia (PANDA, https://www.panda.org.au/).
Apart from giving up your time, we do not expect that there will be any other inconveniences associated with taking part in this study. Travel can be an inconvenience when you are unwell and have a new baby; to minimise this, we are offering home visits.
Who will have access to my information?
The information collected in this research will be non-identifiable (anonymous). No one, not even the research team will be able to identify your information. The following people will have access to the information we collect in this research:
· The research team; and
· In the event of an audit or investigation, staff from the Curtin University Office of Research and Development.
We will ask for your name and contact details when you enrol in the trial, to allow us to contact you to arrange session times. This identifying information will be stored in a separate, password-protected file, accessible by the primary researcher only; it will not be stored or linked with the data we collect during the trial for analysis.
We will also ask for your name in the first part of this survey (the electronic consent form). This will not be stored or linked to the rest of your survey responses, which are anonymous.
All data will be stored electronically on a remote access research drive at Curtin University, which is password-protected and only accessible by the PhD physiotherapist researcher and her supervisors. The information we collect in this study will be kept under secure conditions at Curtin University, for 15 years after the research is published, and then it will be destroyed. The results of this research may be:
· Presented at conferences
· Published in professional journals
· Made available on relevant health websites, news/media outlets and at the recruitment sites.
You will not be identified in any results that are published or presented.
Your breast milk samples will be kept for further analysis after the study. Further analyses may include additional measurements of inflammatory indicators, microbiome analysis, and bacterial profile testing of the milk, which will enhance our understanding of the treatment that was applied. The samples will be kept for a minimum of 15 years after publication.
Will you tell me the results of the research?
If you are interested in obtaining a summary of the results, please provide the researchers with your email address at any time. Results will not be individual but based on all the information we collect and review as part of the research.
Do I have to take part in the research project?
Taking part in a research project is voluntary.
· If you decide to take part and then change your mind, that is okay, you can withdraw from the project.
· If you choose not to take part or start and then stop the study, it will not affect your relationship with the University, researchers, or recruitment site.
· You can leave the study at any time and seek physiotherapy treatment in the private or public system.
You do not have to give us a reason; just tell us that you want to stop. Please let us know you want to stop so we can make sure you are aware of any thing that needs to be done so you can withdraw. We will be unable to remove your information because it has been collected in an anonymous way.
What happens next and who can I contact about the research?
To obtain further information or if you have any questions, please contact either:
Mrs Emma Heron, Primary researcher: Phone: (03) 9481 6312 | Email: emma.duff@postgrad.curtin.edu.au
Dr Leanda McKenna, PhD Supervisor: Phone: (08) 9266 3660 | Email: L.Mckenna@curtin.edu.au
If you decide to take part in this research, we will ask you to sign the consent form. By signing it is telling us that you understand what you have read and what has been discussed. Signing the consent indicates that you agree to be in the research project and have your health information used as described. Please take your time and ask any questions you have before you decide what to do.
Curtin University and Mercy Health Human Research Ethics Committee (HREC) have approved this study (HREC numbers 2021-0315 and 2021-007). Should you wish to discuss the study with someone not directly involved, in particular, any matters concerning the conduct of the study or your rights as a participant, or you wish to make a confidential complaint, you may contact:
Curtin University
Ethics Officer: Phone: (08) 9266 9223
Manager, Research Integrity: Phone: (08) 9266 7093 | Email: hrec@curtin.edu.au
Mercy Health
Administrative Officer, Mercy HREC: Phone: (03) 8458 4808 | Email: ethics@mercy.com.au
